7-Dehydrocholesterol
7-Dehydrocholesterol Specification
| Appearance | Almost white or yellowish crystalline powderr | Standard Packing | 1kgs/bag or per customer request | |
| Assay (HPLC) | ≥ 95% or ≥ 97% | Inventory | Normally we have 7-Dehydrocholesterol in stock | |
| optical activity [α]20D | −116±6°, c = 1% in chloroform | |||
| Melting point | 148-152 °C | 7-Dehydrocholesterol physical parameters | ||
| Loss on drying | ≤ 0.5% | CAS No: | 434-16-2 | |
| Formula | C22H44O | |||
| Molecular Weight | 384.64 | |||
| Synonym | (−)-7-Dehydrocholesterol, 3β-Hydroxy-5,7-cholestadiene, 5,7-Cholestadien-3β-ol, Provitamin D3 | |||
| Structure | 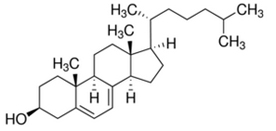 |
|||
| Certificate of Analysis | 7-Dehydrocholesterol COA | |||
| Literature | 7-Dehydrocholesterol literature | |||
| MSDS | 7-Dehydrocholesterol MSDS | |||
| References: | ||||
| 1. |
The Smith-Lemli-Opitz syndrome (SLOS) is a common birth defect–mental retardation syndrome caused by a defect in the enzyme that reduces 7-dehydrocholesterol to cholesterol. Because of this block, patients' plasma cholesterol levels are generally low while 7-dehydrocholesterol concentrations are markedly elevated. In addition, plasma total sterols are abnormally low and correlate negatively with the percent of 7-dehydrocholesterol (r = -0.65, P < 0.0001) suggesting that 7-dehydrocholesterol might inhibit the activity of HMG-CoA reductase. Cultured skin fibroblasts from SLOS patients grown in fetal bovine serum or for 1 day in delipidated medium contain little 7-dehydrocholesterol (3 ± 1% of total sterols) and HMG-CoA reductase activities are indistinguishable from that measured in control cells. However, raising the 7-dehydrocholesterol concentration to 20 ± 3% of total sterols, equal to the mean proportion in plasma of SLOS patients, by either growing cells for 1 week in delipidated medium or adding 20 μg/ml 7-dehydrocholesterol directly to the cells reduced HMG-CoA reductase activities from 74 ± 7 to 9 ± 2 pmol/min per mg protein, or from 92 ± 22 to 16 ± 4 pmol/min per mg protein, respectively (P < 0.01). In contrast, adding 20 μg/ml cholesterol evoked a 2- to 4-fold lesser suppression of activity (39 ± 8 pmol/min per mg protein, P < 0.05, vs. 7-dehydrocholesterol). HMG-CoA synthase and LDL binding were inhibited equally by 7-dehydrocholesterol and cholesterol. Ketaconazole prevented the down-regulation of HMG-CoA reductase by 7-dehydrocholesterol, suggesting that an hydroxylated derivative of 7-dehydrocholesterol may be especially important in suppressing cholesterol synthesis. These results demonstrate that 7-dehydrocholesterol, perhaps as an hydroxylated derivative(s), is a very effective feedback inhibitor of HMG-CoA reductase.—Honda, M., G. S. Tint, A. Honda, L. B. Nguyen, T. S. Chen, and S. Shefer. 7-Dehydrocholesterol down-regulates cholesterol biosynthesis in cultured Smith-Lemli-Opitz syndrome skin fibroblasts. |
|||
| 2. |
Localization and biosynthesis of 7-dehydrocholesterol in rat skinBy differential assay, the concentration of 7-dehydrocholesterol in the dead keratin layer, the epidermal mucosa, the sebaceous glands and associated appendages of the epidermis, and the dermis of rat skin was determined; 85% of the 7-dehydrocholesterol was isolated from slices of skin that surface keratin and sebaceous glands. Slices of skin were incubated with labeled acetate in vitro. Labeled 7-dehydrocholesterol was separated from other labeled skin sterols as the hydrolyzed 5, 8 Diels-Alder adduct. Approximately 80% of the labeled 7-dehydrocholesterol was isolated from slices of rat skin that contained sebaceous glands. The formation of cutaneous 7-dehydrocholesterol was studied in vivo by the injection of labeled acetate. More than 80% of the labeled cutaneous 7-dehydrocholesterol was isolated with the sebaceous tissue. Time-course studies suggested that 7-cholestenol may be a precursor of cutaneous 7-dehydrocholesterol. |
|||
|
Copyright © 2020 - . All Rights Reserved. |
||||