|
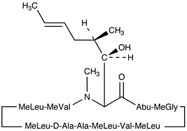
Cyclosporine is a potent immunosuppressive agent that in animals prolongs survival of allogeneic transplants involving skin, kidney, liver, heart, pancreas, bone marrow, small intestine, and lung. Cyclosporine has been demonstrated to suppress some humoral immunity and to a greater extent, cell-mediated immune reactions such as allograft rejection, delayed hypersensitivity, experimental allergic encephalomyelitis, Freund’s adjuvant arthritis, and graft vs. host disease in many animal species for a variety of organs.
The effectiveness of Cyclosporine results from specific and reversible inhibition of immunocompetent lymphocytes in the G0- and G1-phase of the cell cycle. T-lymphocytes are preferentially inhibited. The T-helper cell is the main target, although the T-suppressor cell may also be suppressed. Cyclosporine also inhibits lymphokine production and release including interleukin-2.
No effects on phagocytic function (changes in enzyme secretions, chemotactic migration of granulocytes, macrophage migration, carbon clearance in vivo) have been detected in animals. Cyclosporine does not cause bone marrow suppression in animal models or man.
Pharmacokinetics:
The immunosuppressive activity of Cyclosporine is primarily due to parent drug. Following oral administration, absorption of Cyclosporine is incomplete. The extent of absorption of Cyclosporine is dependent on the individual patient, the patient population, and the formulation. Elimination of Cyclosporine is primarily biliary with only 6% of the dose (parent drug and metabolites) excreted in urine. The disposition of Cyclosporine from blood is generally biphasic, with a terminal half-life of approximately 8.4 hours (range 5 to 18 hours). Following intravenous administration, the blood clearance of Cyclosporine (assay: HPLC) is approximately 5 to 7 mL/min/kg in adult recipients of renal or liver allografts. Blood Cyclosporine clearance appears to be slightly slower in cardiac transplant patients.
The Cyclosporine Capsules USP MODIFIED and Cyclosporine Oral Solution USP MODIFIED are bioequivalent. Cyclosporine Oral Solution USP MODIFIED diluted with orange juice or apple juice is bioequivalent to Cyclosporine Oral Solution USP MODIFIED diluted with water. The effect of milk on the bioavailability of Cyclosporine when administered as Cyclosporine Oral Solution USP MODIFIED has not been evaluated.
The relationship between administered dose and exposure (area under the concentration versus time curve, AUC) is linear within the therapeutic dose range. The intersubject variability (total, %CV) of Cyclosporine exposure (AUC) when Cyclosporine Oral Solution USP MODIFIED or Sandimmune® (Cyclosporine Oral Solution USP) is administered ranges from approximately 20% to 50% in renal transplant patients. This intersubject variability contributes to the need for individualization of the dosing regimen for optimal therapy (see DOSAGE AND ADMINISTRATION). Intrasubject variability of AUC in renal transplant recipients (%CV) was 9% to 21% for Cyclosporine Oral Solution USP MODIFIED and 19% to 26% for Sandimmune® (Cyclosporine Oral Solution USP). In the same studies, intrasubject variability of trough concentrations (%CV) was 17% to 30% for Cyclosporine Oral Solution USP MODIFIED and 16% to 38% for Sandimmune® (Cyclosporine Oral Solution USP).
Cyclosporine, the active ingredient of Cyclosporine Oral Solution USP MODIFIED, can cause nephrotoxicity and hepatotoxicity. The risk increases with increasing doses of Cyclosporine. Renal dysfunction including structural kidney damage is a potential consequence of Cyclosporine Oral Solution USP MODIFIED and therefore renal function must be monitored during therapy. Care should be taken in using Cyclosporine with nephrotoxic drugs
|