|
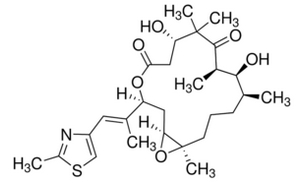
Epothilone B (BMS-247550) is a 16-membered polyketide macrolactone with a methylthiazole group connected to the macrocycle by an olefinic bond. The polyketide backbone was synthesized by type I polyketide synthase (PKS) and the thiazole ring was derived from a cysteine incorporated by a nonribosomal peptide synthetase (NRPS). In this biosythesis, both PKS and NRPS use carrier proteins, which have been post-translationally modified by phosphopantheteine groups, to join the growing chain. PKS uses coenzyme-A thioester to catalyze the reaction and modify the substrates by selectively reducing the β carbonyl to the hydroxyl (Ketoreductase, KR), the alkene (Dehydratase, DH), and the alkane (Enoyl Reductase, ER). PKS-I can also methylate the α carbon of the substrate. NRPS, on the other hand, uses amino acids activated on the enzyme as aminoacyl adenylates. Unlike PKS, epimerization, N-methylation, and heterocycle formation occurs in NRPS enzyme.
Epothilone B starts with a 2-methyl-4-carboxythiazole starter unit, which was formed through the translational coupling between PKS, EPOS A (epoA) module, and NRPS, EPOS P(epoP) module. The EPOS A contains a modified β-ketoacyl-synthase (malonyl-ACP decarboxylase, KSQ), an acyltransferase (AT), an enoyl reductase (ER), and an acyl carrier protein domain (ACP). The EPOS P however, contains a heterocylization, an adenylation, an oxidase, and a thiolation domain. These domains are important because they are involved in the formation of the five-membered heterocyclic ring of the thiazole. EPOS P activates the cysteine and binds the activated cysteine as an aminoacyl-S-PCP. Once the cysteine has been bound, EPOS A loads an acetate unit onto the EPOS P complex, thus initiating the formation of the thiazoline ring by intramolecular cyclodehydration.
Once the 2-methylthiazole ring has been made, it is then transferred to the PKS EPOS B (epoB), EPOS C (epoC), EPOS D (epoD), EPOS E (epoE), and EPOS F (epoF) for subsequent elongation and modification to generate the olefinic bond, the 16-membered ring, and the epoxide, as seen in Figure 5. One important thing to note is the synthesis of the gem-dimethyl unit in module 7. These two dimethyls were not synthesized by two successive C-methylations. Instead one of the methyl group was derived from the propionate extender unit, while the second methyl group was integrated by a C-methyl-transferase domain.
Epothilone B possess the same biological effects as taxol both in vitro and in cultured cells. This is because they share the same binding site, as well as binding affinity to the microtubule. Like taxol, epothilone B binds to the α β-tubulin heterodimer subunit. Once bound, the rate of αβ-tubulin dissociation decreases, thus stabilizing the microtubules. Furthermore, epothilone B has also been shown to induce tubulin polymerization into microtubules without the presence of GTP. This is caused by formation of microtubule bundles throughout the cytoplasm. Finally, epothilone B also causes cell cycle arrest at the G2-M transition phase, thus leading to cytotoxicity and eventually cell apoptosis
Epothilone B could enhance the effects of radiation in human lung cancer cells both in vitro and in vivo and that a G2/M block and increased apoptosis might be possible explanations for the enhancement.[5]
|