Sodium Hyaluronate
Sodium Hyaluronate, Eye drop Specification (Below) . Cosmetic and injection grade is also available.
| Appearance | A white or almost white, very hygroscopic powder or a fibrous aggregate | Standard Packing | 500g/bag or per customer request | |
| Assay (HPLC) | 95.0-105.0% | Inventory | Normally we have Sodium Hyaluronate in stock | |
| pH | 5.0-8.5 | |||
| Nucleic acids | ≤ 0.5 (A260nm) | Sodium Hyaluronate physical parameters | ||
| Protein | ≤ 0.1% | CAS No: | 9067-32-7 | |
| Appearance of solution | ≤ 0.01 (A600nm) | Formula |
(C14H20NNaO11)n |
|
| Iron | ≤ 80 ppm | Synonym | Poly(β-glucuronic acid-[1→3]-β-N-acetylglucosamine-[1→4]), alternating | |
| Loss on drying | ≤ 20% | Structure | 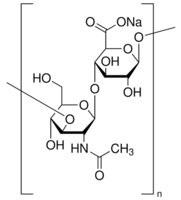 |
|
| Chlorides | ≤ 0.5% | |||
| Microbial contamination | ≤ 100/g | |||
| Bacterial endotoxins | 0.05 IU/mg | |||
| Certificate of Analysis | Sodium Hyaluronate COA | |||
| Literature | Sodium Hyaluronate literature | |||
| MSDS | Sodium Hyaluronate MSDS | |||
| References: | ||||
| 1. | P. Coimbra et al.: International Journal of Biological Macromolecules, Volume 49, Issue 4, 1 November 2011, Pages 573–579. Sodium hyaluronate/chitosan polyelectrolyte complex scaffolds for dental pulp regeneration: Synthesis and characterization | |||
| 2. | K. Ruckmani et al: Journal of Pharmaceutical Analysis, Volume 3, Issue 5, October 2013, Pages 324–329. Determination of sodium hyaluronate in pharmaceutical formulations by HPLC–UV | |||
|
Copyright © 2020 - . All Rights Reserved. |
||||
|
Home >>
|
Product >>
|
Carbohydrate >>
|
Sodium Hyaluronate
|